Use of bevacizumab in patients with mSCLC and no evidence of disease in the lungsUse of bevacizumab in patients with mSCLC and no evidence of disease in the lungs
Editor's comments
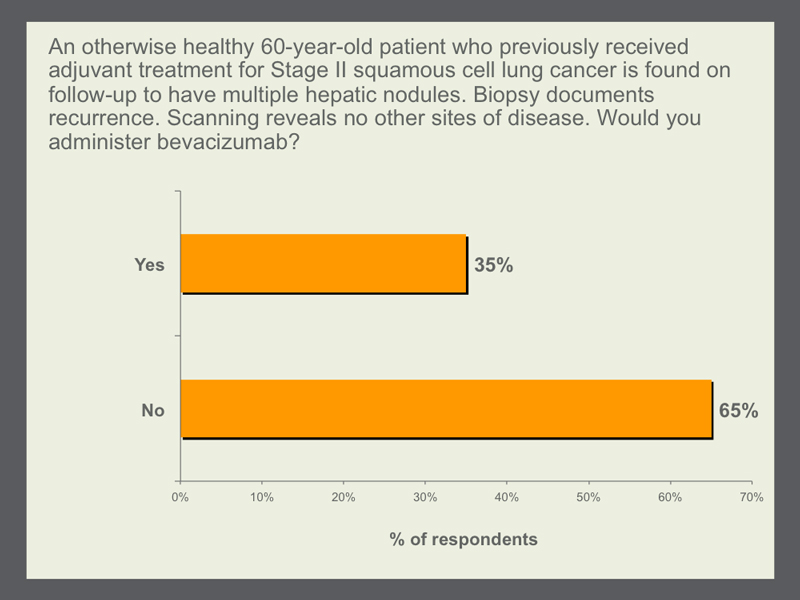
Squamous cell histology has long been considered a contraindication to the use of bevacizumab. However, this thinking relates more to concerns about toxicity, specifically hemoptysis, than it does to efficacy. We decided to try to tease out exactly what was driving the issue by asking about a patient with squamous cell lung cancer who, after resection and adjuvant therapy, experienced a recurrence with disease entirely outside the lung. Most GOs and Dr Ramalingam would not use bevacizumab in this situation, but about one third would, including Dr Wakelee, who points out that patients with squamous cell tumors are safely receiving bevacizumab in her adjuvant ECOG-E1505 study. |