Dr Jeff Sharman talks about novel agents and a new algorithm for the management of CLL…and more
 Less than 2 weeks ago, in a historic act that will instantly affect the treatment of one of the most common hematologic cancers in general oncology practice, the FDA broadened the first-line indication of ibrutinib to now include patients both with and without 17p-deleted chronic lymphocytic leukemia (CLL). This landmark event is just part of an unprecedented explosion of new data and treatment options that have redefined the management of this disease over the past few years.
Less than 2 weeks ago, in a historic act that will instantly affect the treatment of one of the most common hematologic cancers in general oncology practice, the FDA broadened the first-line indication of ibrutinib to now include patients both with and without 17p-deleted chronic lymphocytic leukemia (CLL). This landmark event is just part of an unprecedented explosion of new data and treatment options that have redefined the management of this disease over the past few years.
One of the many investigators in this worldwide effort is Dr Jeff Sharman, who first at Stanford and now in Springfield, Oregon has gained extensive practical experience working with many of the novel agents that are now part of current algorithms. I met with Jeff to learn about his perspectives — from both a clinical and a research standpoint — on relevant CLL and non-Hodgkin lymphoma data sets presented at the December ASH meeting and how these add to the rapidly evolving therapeutic paradigms in these cancers (click for the interview). Below find a summary of this conversation along with slides detailing the key findings from the ASH papers (click to review).
RESONATE-2: Ibrutinib versus chlorambucil in untreated CLL
In what might be compared to a matchup between the Green Bay Packers and my alma mater, Milford Mill High School, this randomized Phase III trial not surprisingly demonstrated the clear-cut superiority of the Bruton tyrosine kinase (BTK) inhibitor, with spectacular progression-free survival (PFS) and overall survival (OS) HRs of 0.16 for both endpoints. Although the trial focused on patients older than 65, the design and the results did not compel the FDA to tie an age stipulation to the recent approval expansion. In addition, ibrutinib had an acceptable safety profile, with the majority of adverse events being Grade 1 and a lower rate of treatment discontinuation due to toxicity compared to chlorambucil (9% versus 23%). As such, many, including Dr Sharman, believe ibrutinib will become the standard first-line treatment for most patients with CLL. He pointed out that the one exception might be younger patients with IgVH-mutated disease without high-risk FISH findings in whom a chemoimmunotherapy regimen like fludarabine/cyclophosphamide/rituximab (FCR) could potentially result in a prolonged remission or cure without the need for continuous treatment.
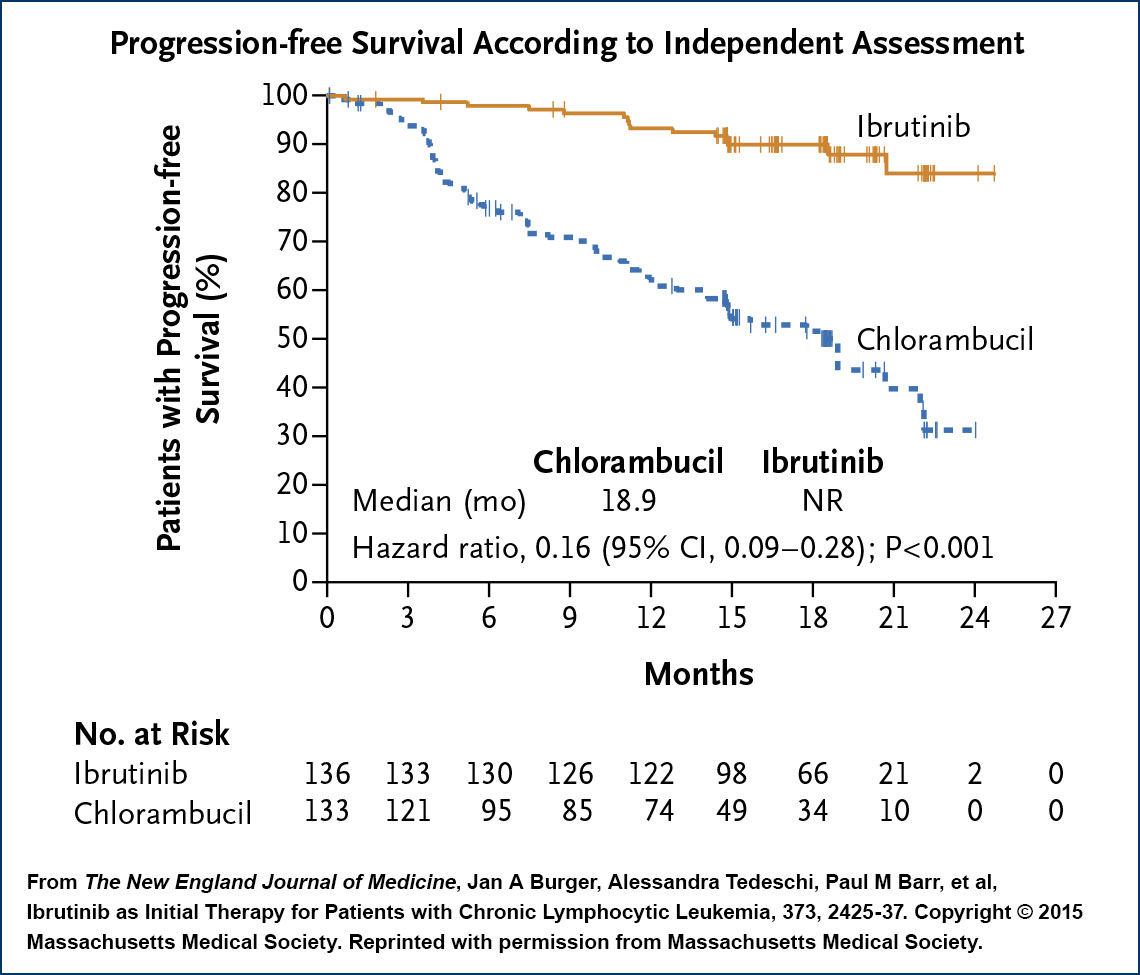 |
In discussing how the indefinite use of up-front ibrutinib may affect clinical practice, Dr Sharman focused on the need to pay even greater attention to the variety of treatment-related complications and potential adverse drug-drug interactions (discussed in another ASH report) that might lead to temporary discontinuation of this generally well-tolerated therapy. His concern in this regard stems from recent research demonstrating that treatment interruption for even 8 days has been associated with a reduction in PFS, a cautionary tale about the potential challenges of administering this or almost any agent indefinitely.
Acalabrutinib (acala-B): A better BTK inhibitor?
The extraordinary efficacy of acala-B might or might not exceed that of ibrutinib, but perhaps of equal importance is the hope that the safety profile may be superior, with early-onset headaches being the most common problem rather than the arthralgias, bruising, bleeding and atrial fibrillation observed with ibrutinib. In a Phase I/II trial of 60 evaluable patients with relapsed disease, responses were observed in 95% of patients, including all 18 with del(17p). A head-to-head Phase III trial will compare these relatively close cousins, but Dr Sharman believes this is another novel agent churning its way steadily toward approval.
Idelalisib in CLL
In one of the highlights of the late-breaking abstract session, Dr Andrew Zelenetz presented results of a Phase III trial evaluating bendamustine/rituximab (BR) alone or with idelalisib in relapsed/refractory (R/R) disease. The study, which was stopped early based on “overwhelming efficacy,” demonstrated an impressive improvement in both PFS (HR 0.33) and OS (HR 0.55), and the findings were consistent for patients with or without high-risk features. Although Dr Sharman questions whether bendamustine really adds much to idelalisib/rituximab, he does consider BR/idelalisib a rational option in the R/R setting.
Importantly, another study at ASH — idelalisib up front combined with ofatumumab — revealed an overall response rate (ORR) of 100% among 24 patients but an excess of immune-related adverse events such as hepatitis, pneumonitis and colitis. Dr Sharman believes these toxicities are likely related to the drug’s inhibition of regulatory T cells and explained that the lower frequency of idelalisib-related immune toxicity in the R/R setting may be the result of what he calls “immuno-plegia” as a consequence of prior therapies such as FCR. As a direct result of these new toxicity findings, just yesterday the FDA announced that 6 ongoing combination trials involving idelalisib and a number of other anti-cancer agents had been stopped.
Venetoclax in CLL
Although it is still in clinical development, the emergence of this highly effective Bcl-2 inhibitor represents another gigantic recent CLL advancement, and in Orlando we saw more impressive data with this agent in del(17p) disease with an ORR of 79.4% among 107 patients with R/R disease. We were also treated to some encouraging findings with combination regimens that included bendamustine, rituximab and obinutuzumab (obin) administered prior to, after or with the Bcl-2 inhibitor.
The FDA has now bestowed not 1 but 2 breakthrough therapy designations upon the drug in CLL, and Dr Sharman believes that the overwhelming database supporting the useful clinical role of this agent will likely lead to its approval this year. However, he and others remain somewhat concerned about the potential for tumor lysis syndrome (TLS), which was observed in early trials, and would like to see specific recommendations emerge for how to prevent or manage this phenomenon. In that regard, he believes that we will likely be assessing pretreatment tumor burden and renal function to determine baseline risk for TLS and then aggressively monitoring or even hospitalizing select patients. With that being said, the availability of another profoundly effective agent with a totally different mechanism of action has the entire CLL investigator community wide eyed with excitement over the possibility of long-term or lifelong disease control.
Obinutuzumab in CLL
It will be interesting to see how the role of this novel anti-CD20 antibody evolves with ibrutinib rapidly moving in on its indicated turf and the current/emerging treatment roster loaded with other novel small agents. Regardless, many believe this drug has good activity in CLL (likely more than rituximab) and for that reason were excited to determine how it would fare in combination with bendamustine. At ASH we saw more from the single-arm GREEN study of bendamustine/obin in untreated and R/R disease demonstrating encouraging efficacy and acceptable toxicity with some concerns about TLS and neutropenia. In the current analysis of 158 patients with previously untreated disease, the bendamustine/obin combination yielded a complete response rate of 32.3%, with minimal residual disease negativity in blood and bone marrow of 58.9% and 28.5%, respectively. Despite these data, Dr Sharman believes the regimen is not yet ready for clinical practice in CLL. It is worth noting that on February 26 of this year the FDA approved bendamustine/obin followed by obin maintenance for patients with follicular lymphoma (FL) who did not respond to a rituximab-containing regimen.
Pembrolizumab in CLL with Richter’s transformation (RT)
RT occurs in approximately 2% to 10% of all CLL cases, and Dr Sharman commented that recently 2 patients in his practice died within 72 hours of diagnosis, in keeping with the often rapidly progressive course. In this preliminary ASH report of 5 patients with RT, 4 had mostly rapid and deep responses after receiving this anti-PD-1 antibody alone. Although it is clearly early days, Jeff found the findings so encouraging that he recently initiated treatment outside a trial setting with this agent in a patient with RT.
ASH disappointments in indolent lymphoma (ibrutinib, venetoclax)
With all the excitement in CLL, the ASH papers focused on indolent lymphomas were a bit underwhelming. In particular 2 studies in FL combining ibrutinib with either rituximab or rituximab/lenalidomide (R2) along with a couple of Phase I trials of venetoclax alone or with BR failed to inspire Dr Sharman, particularly when he considers how active idelalisib has been in this setting. He did find intriguing the significant activity observed with the Bcl-2 inhibitor in mantle-cell lymphoma, perhaps related to its biologic and therapeutic similarity to CLL.
Next on this series, Dr Michelle Fanale comments on other lymphoma papers from ASH, including more on the exciting findings now being reported with the use of checkpoint inhibitors in Hodgkin lymphoma.
Neil Love, MD
Research To Practice
Miami, Florida

 Less than 2 weeks ago, in a historic act that will instantly affect the treatment of one of the most common hematologic cancers in general oncology practice, the FDA broadened the first-line indication of ibrutinib to now include patients both with and without 17p-deleted chronic lymphocytic leukemia (CLL). This landmark event is just part of an unprecedented explosion of new data and treatment options that have redefined the management of this disease over the past few years.
Less than 2 weeks ago, in a historic act that will instantly affect the treatment of one of the most common hematologic cancers in general oncology practice, the FDA broadened the first-line indication of ibrutinib to now include patients both with and without 17p-deleted chronic lymphocytic leukemia (CLL). This landmark event is just part of an unprecedented explosion of new data and treatment options that have redefined the management of this disease over the past few years.