Slide 2 of 14
DR WINER: This is the vexing question that has plagued oncologists and patients to some extent more than any others since the adjuvant trastuzumab data were reported. Virtually no patients who had tumors smaller than one centimeter with negative lymph nodes were entered in the trials. In the CIRG trial, almost 30 percent of the patients had negative lymph nodes. HERA had close to 30 percent. In the NSABP trial there were no patients with negative lymph nodes, and the North Central/Intergroup trial had fewer than 15 percent.
I don’t believe that any of us question the potential benefit of trastuzumab in this setting — the benefit of trastuzumab has been consistent across all patient groups. Generally speaking, there is a reduction in the risk of recurrence in the range of 50 percent, maybe a little bit less than that as the studies mature. The big questions for these patients with small tumors are what is the event rate and how big a risk do they face?
Slide 5 of 14
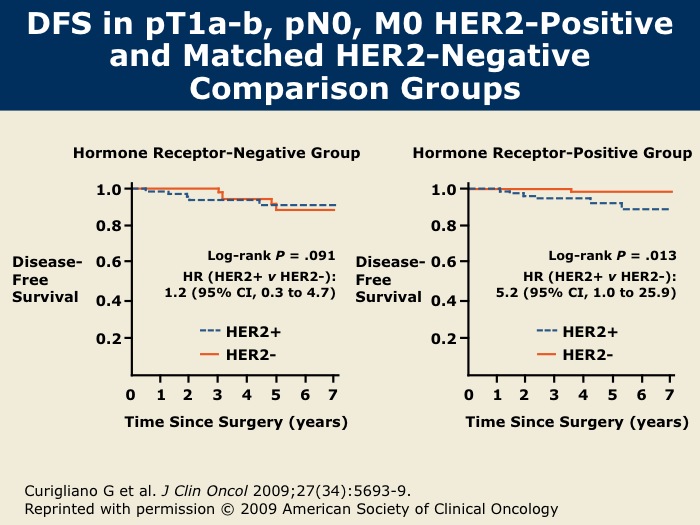
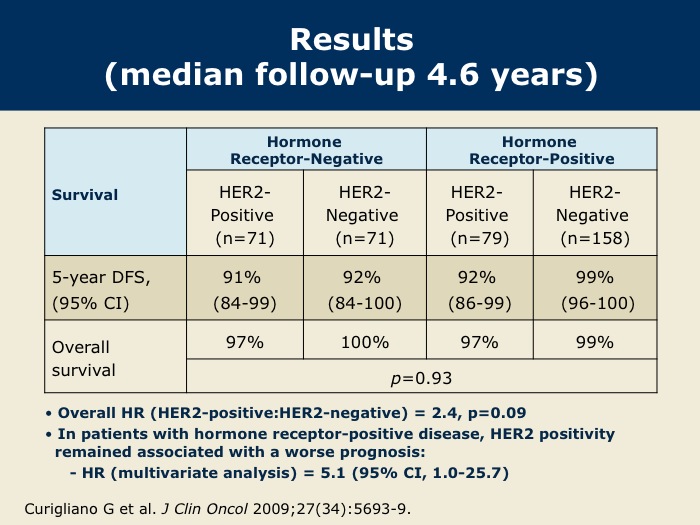
DR WINER: This study, conducted by the European School of Oncology in Milan, was recently published in the Journal of Clinical Oncology in December of 2009. The event rate was in the range of eight to nine percent and was actually similar for patients whether they had ER-positive or ER-negative disease.
Slide 8 of 14
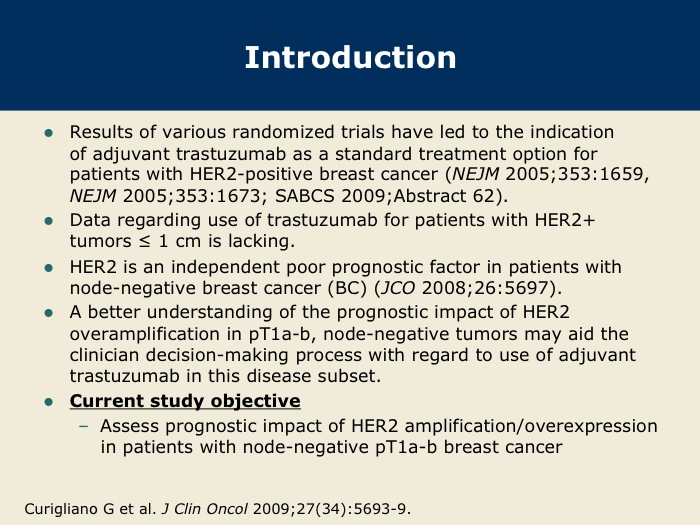
DR GOSS: The question exists as to how to manage clinically early-stage breast cancer that is HER2-positive with small primary tumors. We don’t know the long-term natural history of HER2-positive breast cancer. We know that it’s a marker of early recurrence and higher recurrence risk in both ER-positive and ER-negative breast cancer, but we don’t know the annual hazard of recurrence or the cumulative risk recurrence rate.
The FDA approved trastuzumab initially for patients with node-positive, HER2-positive breast cancer, based on trial results in which the majority of patients had node-positive disease. After the results of the HERA trial the regulatory authorities expanded the indication worldwide to include patients with node-negative breast cancer with tumors larger than one centimeter.
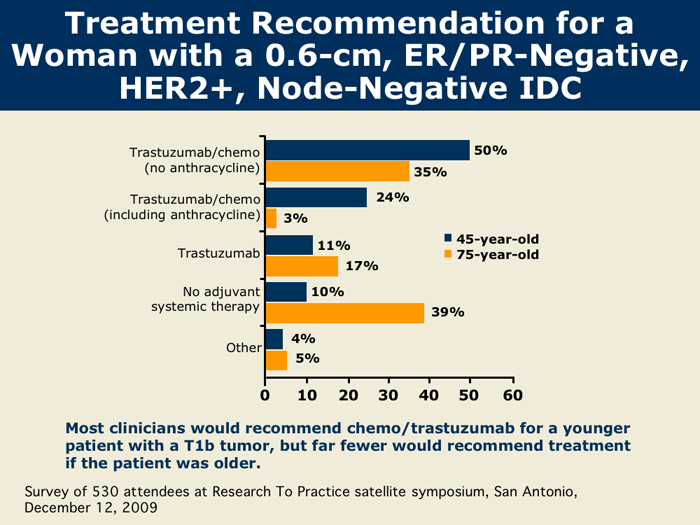
However, many physicians and patients asked: If my tumor is smaller than one centimeter and HER2-positive, what is my risk, and does it merit aggressive therapy with either systemic chemotherapy or anti-HER2 therapy?
Slide 9 of 14
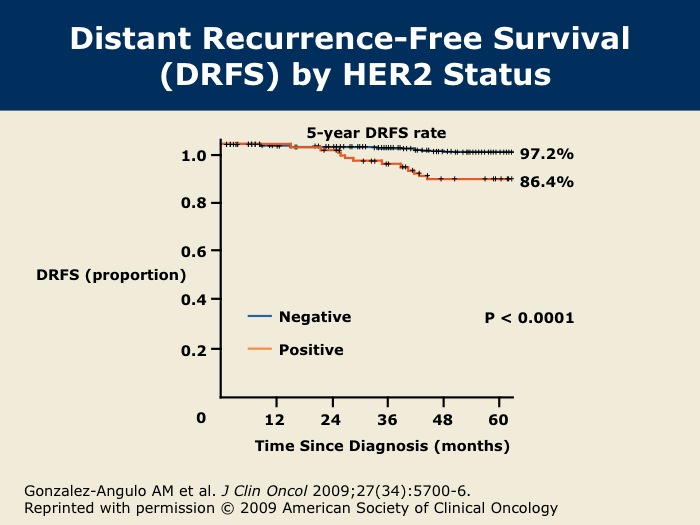
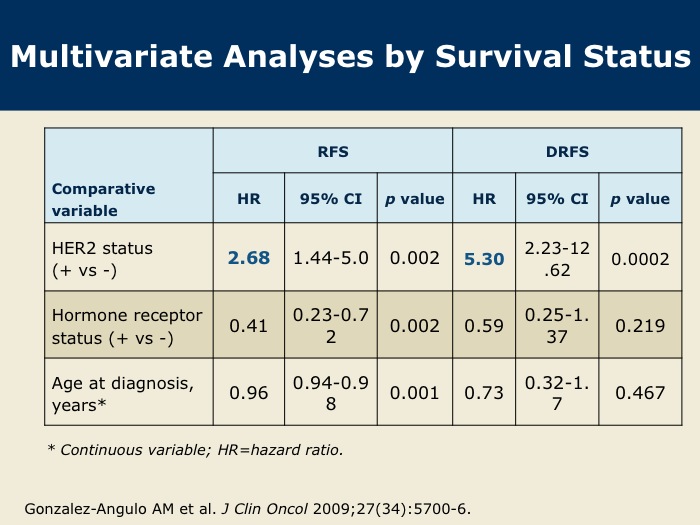
DR WINER: These data were presented at the 2008 San Antonio Breast Cancer Symposium and recently published in the Journal of Clinical Oncology. This was a retrospective analysis from MD Anderson on 965 total patients with T1a and b (ie, tumors one centimeter or smaller), node-negative, HER2-positive breast cancer. Roughly 10 percent of these patients had HER2-positive disease, so about 100 patients. The median follow-up was 74 months, and there were a total of 72 disease recurrences at five years.
Slide 10 of 14
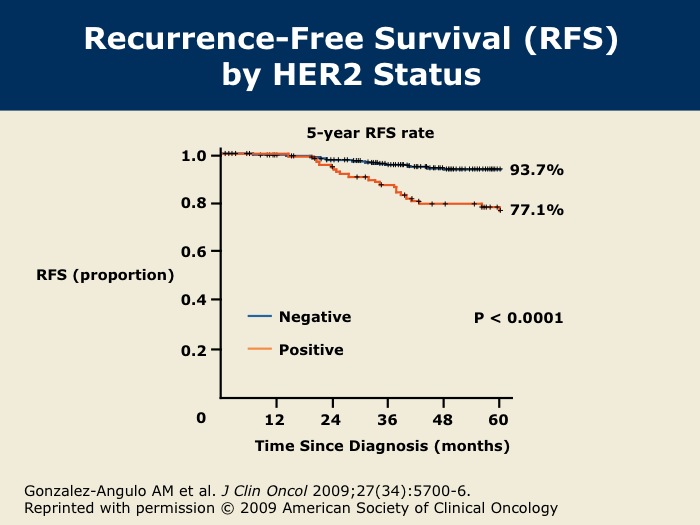
DR WINER: The five-year disease-free survival was 77 percent for patients with HER2-positive disease and 94 percent for patients with HER2-negative disease.
DR GOSS: Dr Gonzalez-Angulo and colleagues from MD Anderson show that HER2-positive tumors smaller than or equal to one centimeter have a five-year risk of recurrence of up to 23 percent. Our group at Massachusetts General Hospital has published that there is a 10 percent rate of recurrence over five years, cumulatively, for HER2-positive tumors smaller than one centimeter in size. There’s a spectrum in the rate of recurrence, but either way, these patients seem to have a higher risk of recurrence than non-HER2-matched controls, and it’s worrisome.
Slide 13 of 14
DR GIANNI: We try to treat node-negative, HER2-positive, small tumors with trastuzumab to take advantage of the fact that in the HERA trial we had 30 percent of all patients with node-negative breast cancer and, overall, there was no difference in the effect of trastuzumab on disease-free survival for those patients than for those with node-positive breast cancer. If the tumor is very small, I would offer the opportunity of a treatment containing trastuzumab in a young woman.